The vast and rapidly expanding body of health-related data creates challenges. Providers and patients alike are faced with more information than they can process in a reasonable amount of time. Health information technology (IT) has potential to help providers, patients, and healthcare systems quickly access and effectively use clinical knowledge and patient-specific data. Massive investments in health IT have led to nearly universal implementation of electronic health records (EHRs) in U.S. hospitals and medical practices; (1,2) however, the benefits for clinical care have fallen short of expectations. (3) Additional investment and commitment are needed to create more effective health IT systems and tools—including but not limited to EHRs—to facilitate high-quality personalized care.
Suboptimal application of the evidence-based clinical practice guidelines—including guidelines for cancer risk assessment and screening—is a critical problem that should be addressed through health IT. Machine-interpretable representations of clinical guidelines—or computable guidelines—could be used to create health IT tools, including clinical decision support (CDS), that allow providers and patients to quickly determine what care is recommended based on patient-specific factors. Cancer screening is particularly well suited to benefit from health IT, including computable guidelines and CDS, for multiple reasons:
- Screening eligibility should be widely and repeatedly assessed—Virtually all adults will be eligible for screening for one or more cancers over the course of their lives. Screening tests must be repeated on a regular basis to improve outcomes. Furthermore, recommendations must be revisited repeatedly because each person’s risk factors (e.g., family history, smoking history) and health history (e.g., diagnoses, results of prior screening tests) change over time and guidelines are updated based on new evidence. Algorithms that can be run automatically and modified as guidelines evolve would help providers and healthcare systems more quickly and efficiently mine records to identify patients eligible or overdue for screening.
- Screening guidelines are increasingly complex—Many factors are taken into account when assessing screening eligibility (see Cancer Screening and Genetic Testing Recommendations May Be Affected By). Guidelines are likely to become increasingly complex as guideline makers incorporate additional factors (e.g., breast density for breast cancer) that help determine an individual’s cancer risk. In some cases, patients and providers also must weigh the pros and cons of different modalities available to screen for a given cancer (e.g., colonoscopy and fecal immunochemical test [FIT] for colorectal cancer). The recommended frequency of screening often differs based on the screening modality and individual factors. CDS can integrate person-specific information from multiple sources and present it to patients and providers in ways that facilitate assessment and shared decision-making.
- Screening is a multistep process—A provider recommendation for cancer screening is only the first step. Cancer screening often includes additional appointments at outside facilities. Timely follow-up, additional testing, and/or a modified schedule for future screening may be needed based on the result of each screen. Management of abnormal results in turn requires consideration of additional clinical guidelines and care recommendations. Health IT tools can be used to monitor initiation and completion of the screening process, as well as receipt of follow-up care, for individuals and groups of patients. CDS can incorporate multiple sets of screening and follow-up guidelines to ensure seamless care management. Health IT also can facilitate communication and handoffs among healthcare team members.
The Panel recommends creation of computable guidelines for cancer screening and use of these guidelines to create CDS for cancer risk assessment, screening, and follow-up care.
Recommendation 4.1: Create computable versions of cancer screening and risk assessment guidelines.
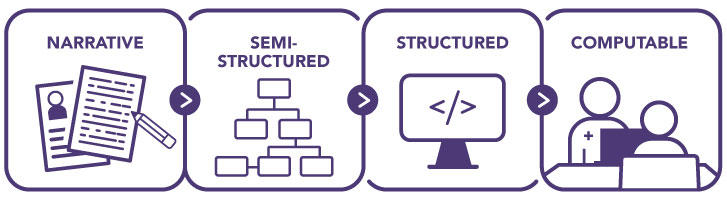
Cancer risk assessment, screening, and follow-up guidelines are issued by several organizations (Table 1) and are disseminated in narrative form to healthcare providers and systems through publication in peer-reviewed journals, organizational websites, and professional societies. Before being incorporated into health IT tools—including CDS or other tools—narrative guidelines must be converted to a more structured format (Figure 7). For automated tools, computable guidelines—a format that can be fully interpreted and executed by a computer—must be created. Currently, each health IT developer using a guideline independently renders a computable representation. This duplicative process is an inefficient use of resources that must be repeated every time guidelines are updated and can result in unintentional variability in guideline interpretation and implementation.
Figure 7: Development of Computable Guidelines
Development of health IT tools would be more efficient if all cancer screening guidelines were publicly available in a computable format. Computable guidelines created using open-access data standards, such as Fast Healthcare Interoperability Resources (FHIR), (4) are platform agnostic and could be readily used by health IT developers to create tools to support clinicians, healthcare systems, and patients. Tools could include CDS (see Recommendation 4.2), as well systems for quality measurement and reporting, generation of case reports, and creation of population registries. In addition to saving resources, the availability of computable guidelines would promote broader, more consistent, and faster implementation of cancer screening guidelines.
Standards, methods, and tools for translating guidelines to computable formats are actively being developed and refined (see Data and Exchange Standards). (4,5,6) Creation of computable guidelines requires the expertise of a variety of informaticians capable of translating technical medical information into advanced logic that can be understood by computer systems. Ideally, informaticians would interface with guideline makers, clinical domain experts, and health IT developers to ensure that the programmed terminology and logic are accurate as well as usable and valid for downstream applications. Proactive collaboration between informaticians and guideline makers during the guideline development process can help identify unintentional gaps or lack of clarity in recommendations; addressing these issues through an iterative process can both strengthen the recommendations and facilitate translation to a computable form.
Resources are needed to support these collaborations and catalyze generation of computable guidelines for cancer risk assessment and screening. Guideline makers with access to the necessary resources and expertise should incorporate creation of computable guidelines into their guideline development process. However, the Panel recognizes that many guideline-making organizations currently do not have the expertise or resources to make their guidelines computable. Research funding organizations with an interest in healthcare quality and implementation—including the Agency for Healthcare Research and Quality (AHRQ), Centers for Disease Control and Prevention (CDC), National Institutes of Health (NIH), American Cancer Society, and others—should fund development of computable guidelines for cancer risk assessment and screening. This could be done through grants to guideline organizations, researchers, or collaborative teams. Alternatively, computable guidelines could be directly created through targeted initiatives of federal agencies (see Making Cancer Screening Guidelines Computable). CDC and AHRQ should consider investment in dedicated programs to support creation of computable guidelines relevant to risk assessment, screening, and follow-up care for cancer and other diseases. Computable guidelines should be shared through public resources, such as the AHRQ CDS Connect Repository, to facilitate their dissemination and use. (7,8)
Recommendation 4.2: Create and deploy effective clinical decision support tools for cancer risk assessment and screening.
CDS can help providers and patients access and integrate clinical knowledge and patient-specific data to guide care (see Tools to Facilitate Clinical Decision-Making). CDS tools are not intended to replace provider judgment or patient decision-making; rather, they are intended to inform and facilitate care. Effective CDS would help alleviate the pressures on providers; they may be particularly beneficial for primary care providers, who are expected to address a wide range of issues within a limited time during appointments, and providers in settings with limited financial resources (e.g., Federally Qualified Health Centers, private practices). Automated CDS also could help reduce the impact of provider bias and ensure that cancer risk assessment is completed and screening recommendations are delivered to all populations.
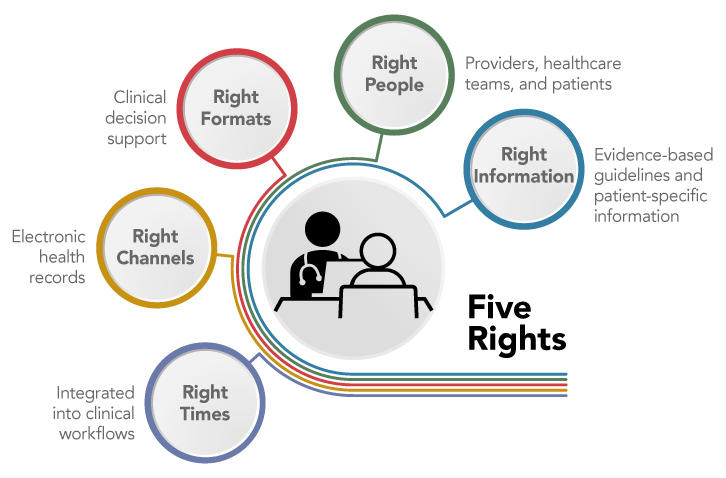
Most EHR systems employ CDS to some extent, often through best practice alerts to providers. While these alerts can improve the safety and quality of care, low-quality alerts can lead to alert fatigue and even interfere with patient care. (9) To effectively improve care, CDS must follow the Five Rights model: the right information must be delivered to the right people in the right formats, through the right channels, and at the right times in the clinical workflow (Figure 8). (10) The key to good CDS begins with the right information; CDS must integrate patient-specific information with evidence-based guidelines (see Recommendation 4.1) and clinical best practices. Usability is key to the success of CDS. CDS for providers must be seamlessly integrated into clinical workflows and provide information in concise, understandable, and actionable formats. CDS also can be created to help inform patient decision-making and allow patients to securely share personal information with healthcare providers as desired; it is critical that information and questions included in patient-facing tools are presented in language that is easy to understand and apply.
Figure 8: Five Rights of Clinical Decision Support
Currently, many healthcare organizations develop and implement their own CDS in parallel, resulting in redundant effort and expense. Progress in development and adoption of standards for clinical data, data exchange, and CDS is providing opportunities to create a collection of shareable, scalable CDS that can easily be implemented or adapted for use in a variety of healthcare settings, including large healthcare systems and small independent practices. (11,12) Health IT developers should use available standards (see Data and Exchange Standards) to the extent possible and build on the knowledge generated through development and implementation of earlier CDS. Evaluation of CDS is needed to measure impact on health outcomes, quality of care, safety, cost, patient satisfaction, and physician productivity. The results should inform improvements in systems and processes to maximize benefit for patients, providers, and healthcare systems.
CDS can be created by EHR vendors, healthcare systems, or third parties, such as academic researchers, patient advocacy organizations, or professional societies. Collaborative approaches that include multiple stakeholder groups and perspectives also may be beneficial. EHR vendors, healthcare organizations, and research funding organizations—including AHRQ, NIH, CDC, and private foundations—should prioritize support for development and evaluation of standards-based, interoperable CDS for cancer risk assessment and screening. The reach of CDS would improve if developers shared code for their tools. This would provide opportunity for institutions with fewer resources—including small practices or healthcare settings with limited resources—to insert existing tools into their EHRs and customize them to meet their needs. The Panel encourages sharing of CDS, such as through the AHRQ CDS Connect Repository; (7) sharing should be a prerequisite for any CDS created using public funds.
CDS should be integrated with EHRs to optimize workflow, facilitate data exchange, and avoid duplicate data entry. EHR vendors should include CDS for cancer risk assessment and screening in standard EHR systems and make it as easy as possible for CDS developed by others to be integrated with the EHR. To this end, it is critical that EHR vendors and IT developers continue to pursue interoperability of health IT systems (see Data Sharing and Interoperability).
Sources
Data and Exchange Standards Sources: HL7 International. HL7 FHIR [Internet]. Ann Arbor (MI): HL7 International; 2019 Nov 1 [cited 2021 Sep 20]. [Available Online]. HL7 International. FHIR clinical guidelines implementation guide [Internet]. Ann Arbor (MI): HL7 International; [updated 2021 Feb 11; cited 2021 Sep 20]. [Available Online]. HL7 International. SMART App Launch Framework [Internet]. Ann Arbor (MI): HL7 International; 2018 Nov 13 [cited 2021 Sep 21]. [Available Online]. Office of the National Coordinator for Health Information Technology. Fed Regist. 2020 May 1;85:25642-961. [Available Online]. Institute of Medicine. Patient safety: achieving a new standard for care. Washington (DC): The National Academies Press; 2004. [Available Online]
Making Cancer Screening Guidelines Computable Sources: Centers for Disease Control and Prevention. Adapting Clinical Guidelines for the Digital Age [Internet]. Atlanta (GA): CDC; [updated 2021 Jul 8; cited 2021 Oct 2]. [Available Online]. HL7 International. FHIR clinical guidelines implementation guide [Internet]. Ann Arbor (MI): HL7 International; [updated 2021 Feb 11; cited 2021 Sep 20]. [Available Online]
Tools to Facilitate Clinical Decision-Making Sources: The Nudge Unit. Home page [Internet]. Philadelphia (PA): Penn Medicine; [cited 2021 Sep 25]. [Available Online]. Hsiang EY, et al. JAMA Netw Open. 2019;2(11):e1915619. [PubMed Abstract]. Orlando LA, et al. BMC Health Serv Res. 2020;20(1). [PubMed Abstract]
Data Sharing and Interoperability: National Academy of Medicine. Taking action against clinician burnout: a systems approach to professional well-being. Washington (DC): The National Academies Press; 2019. [Available Online]. Office of the National Coordinator for Health Information Technology. Fed Regist. 2020 May 1;85:25642-961. [Available Online]. Office of the National Coordinator for Health Information Technology. Strategy on reducing regulatory and administrative burdens relating to the use of health IT and EHRs. Washington (DC): ONC; 2020 Feb. [Available Online]. Office of the National Coordinator for Health Information Technology. Trusted Exchange Framework and Common Agreement [Internet]. Washington (DC): ONC; [cited 2021 May 14]. [Available Online]. Office of the National Coordinator for Health Information Technology. About ONC's Cures Act Final Rule [Internet]. Washington (DC): ONC; [cited 2021 May 14]. [Available Online]
Figure 7 Source: Adapted from: Boxwala AA, et al. J Am Med Inform Assoc. 2011;18:i132-9. [PubMed Abstract]
Figure 8 source: Sirajuddin AM, et al. J Healthc Inf Manag. 2009;23(4):38-45. [PubMed Abstract]