Recommendation 5. Ensure that FDA has appropriate resources to assess cancer drug safety and efficacy efficiently.
The U.S. Food and Drug Administration (FDA) plays a critical role in ensuring patient access to innovative cancer drugs. FDA has been characterized by some as “slow and burdensome,”1 but these claims are unwarranted. FDA reviews and approves drugs more quickly than its European counterpart2 and has cut review times in half over the past 25 years (Figure 5).3 Cancer drugs are no exception—half of new drug applications for cancer treatments approved by FDA between 2003 and 2016 were approved within six months, and virtually all were approved within one year.4
Figure 5
FDA Median Time to Approval for New Drug Applications and Biologics License Applications, Fiscal Years 1993-2016
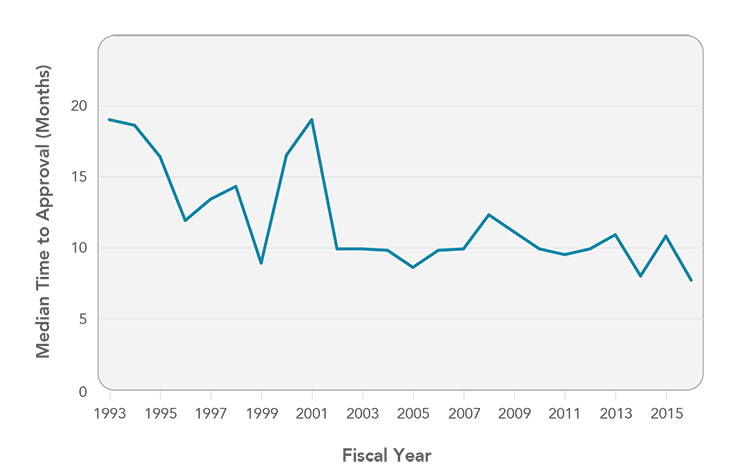
Source: Jenkins JK. CDER new drug review: 2016 update. Presented at: FDA/CMS Summit; 2016 Dec 14; Washington, DC. London (UK): Informa Life Sciences. Available from: https://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/UCM533192.pdf
Cancer drug development and evaluation present distinct challenges, particularly in the age of precision medicine. FDA has implemented policies and programs to address many of these challenges. The Oncology Center of Excellence was established to enable more efficient and effective review of cancer treatments (see FDA Oncology Center of Excellence).5 The Panel supports the efforts of the Center. The agency also has implemented various programs, including breakthrough therapy designation, that allow it to focus resources on particularly promising new drugs to treat serious conditions that may demonstrate substantial improvement over existing therapies.6 In some cases, these programs have helped patients gain earlier access to effective new drugs.7
The Panel urges the President and Congress to ensure that the FDA has the resources and authority to assess the safety and efficacy of oncology products and to appropriately staff the Oncology Center of Excellence. Adequate resources also are needed to conduct postapproval drug safety monitoring, ensure that foreign and domestic manufacturing facilities adhere to safety and quality standards, and enable efficient review of both novel and generic/biosimilar drugs (Recommendation 4).
An adequately staffed and well-resourced FDA is more important than ever in the modern era of oncology product development. Innovative trial designs—such as seamless expansion cohort designs and platform trials—are being developed to evaluate emerging cancer treatments, including molecularly targeted therapies, immunotherapies, and combination therapies. Such trials enable adequate safety and efficacy testing with fewer patients and shorter timeframes than traditional randomized controlled trials. The Panel heard from many stakeholders that FDA regulators and statisticians are at the forefront of clinical trial design and statistical analysis and, as such, are essential assets to cancer product development.
A highly skilled FDA workforce also is essential as the agency considers important questions about incorporation of new kinds of data into its review processes. As directed in the 21st Century Cures Act8 and the FDA Reauthorization Act (FDARA),9 FDA also is working to enhance the patient voice in drug development. The Oncology Center of Excellence is contributing to these efforts through its Patient-Focused Drug Development program (see FDA Oncology Center of Excellence). The Panel commends FDA’s efforts to incorporate patients’ perspectives and experiences in the drug testing and regulatory review process and looks forward to continued commitment to patient-focused drug development.
The 21st Century Cures Act and FDARA also charge the Secretary of the U.S. Department of Health and Human Services and FDA with exploring use of real-world evidence—defined as data from sources other than traditional trials8—in regulatory decision making. Some have expressed concern that this could lead to less rigorous review.10 The Panel agrees it is critical that FDA continue to demand rigorous science for the demonstration of both safety and efficacy. Real-world evidence has potential to offer valuable insights based on how drugs are used and work in clinical settings (see the Panel’s 2016 report Improving Cancer-Related Outcomes with Connected Health). It is important, however, to ensure that data limitations are well characterized and accounted for in statistical analyses and interpretation. Future guidance from FDA on use of real-world evidence should reflect these considerations.
FDA Oncology Center of Excellence
The FDA Oncology Center of Excellence was created in 2016 as part of the Cancer Moonshot with the goal of expediting the development of oncology and hematology medical products. The Center brings together regulatory scientists and reviewers with expertise in drugs, biologics, devices, and data science to support an integrated approach to evaluation of products for the diagnosis and treatment of cancer.
One of the Center’s key efforts is the Patient-Focused Drug Development program. The overarching goal of the program is to identify rigorous methods to assess patients’ experiences to inform evaluation of cancer drugs. Key activities include engaging with patients and patient advocacy groups, fostering research into measurement of patients’ experiences, and generating science-based recommendations for regulatory policy.
References
- McGinley L. Trump calls the FDA "slow and burdensome," but it's faster than ever. The Washington Post [Internet]. 2017 Mar 3 [cited 2017 Mar 9]. Available from: https://www.washingtonpost.com/news/to-your-health/wp/2017/03/02/trump-calls-the-fda-slow-and-burdensome-but-its-faster-than-ever
- Downing NS, Zhang AD, Ross JS. Regulatory review of new therapeutic agents - FDA versus EMA, 2011-2015. N Engl J Med. 2017;376(14):1386-7. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28379798
- U.S. Food and Drug Administration. CDER approval times for priority and standard NDAs and BLAs: calendar years 1993-2016. Silver Spring (MD): FDA; 2016 Dec 31. Available from: https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/NDAandBLAApprovalReports/UCM540942.pdf
- Friends of Cancer Research. Comparison of FDA and EMA review of oncology drugs (2004-2016). Washington (DC): FOCR; 2016 Dec 31. Available from: https://www.focr.org/sites/default/files/pdf/FDA-EMA-findings-summary-12.31.16.pdf
- U.S. Food and Drug Administration. Oncology Center of Excellence [Internet]. Silver Spring (MD): FDA; [updated 2017 Jun 26; cited 2017 Jul 28]. Available from: https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/OCE/default.htm
- U.S. Food and Drug Administration. Fast Track, Breakthrough Therapy, Accelerated Approval, Priority Review [Internet]. Silver Spring (MD): FDA; [updated 2015 Sep 14; cited 2017 Jul 27]. Available from: https://www.fda.gov/ForPatients/Approvals/Fast/default.htm
- Chambers JD, Thorat T, Wilkinson CL, Neumann PJ. Drugs cleared through the FDA's expedited review offer greater gains than drugs approved by conventional process. Health Aff (Millwood). 2017;36(8):1408-15. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28784733
- 114th Congress. 21st Century Cures Act (H.R. 34). 2016 Dec 13. Available from: https://www.congress.gov/bill/114th-congress/house-bill/34
- U.S. Food and Drug Administration. PDUFA reauthorization performance goals and procedures fiscal years 2018 through 2022. Silver Spring (MD): FDA; 2016 Jul 15. Available from: https://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM511438.pdf
- Mazer D, Curfman G. 21st Century Cures Act lowers confidence in FDA-approved drugs and devices. Health Affairs Blog [Internet]. 2017 Feb 14 [cited 2017 Mar 13]. Available from: http://healthaffairs.org/blog/2017/02/14/21st-century-cures-act-lowers-confidence-in-fda-approved-drugs-and-devices
